
Principle
The separation of antibodies from various biomasses can be accomplished using hydrophobic charge induction chromatography with 4-mercapto-ethyl-pyridine as the ligand. Binding is based on mild hydrophobic contact and is accomplished in near-physiological circumstances, without the use of salts. When the pH is reduced, the ligand and the antibody gain a net positive charge under mild acidic circumstances (pH 4.0-4.5).
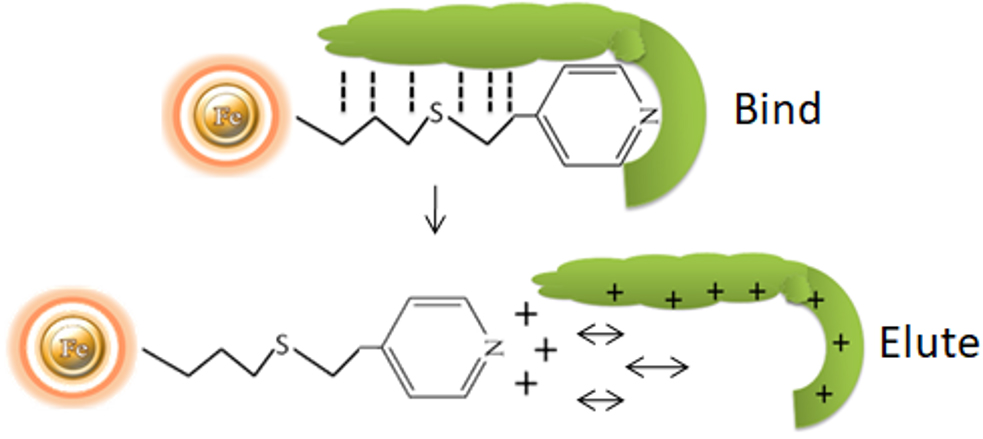
HCIC has several process advantages over more traditional antibody purification methods, such as protein A or ion exchange:
●
Sample preparation is reduced to scarification since feedstocks may be applied without ionic strength or pH adjustment.
●
Preconcentration of dilute samples is unnecessary because effective capture is accomplished even with feedstocks as dilute as 40μg of antibody per ml.
●
Gentle, pH-controlled elution with dilute buffer lowers the possibility of antibody aggregation at lower pH and avoids the requirement for IgG desalting or diafiltration.
Magnetic resins have significant advantages over traditional chromatography, such as column, agarose, or non-magnetic resin. The magnetic bead-based format enables rapid high-yield processing of 96 samples in about 20 minutes, achieving more than 90% purities and recoveries of more than 95% for various IgG species. When using column-based technologies, processing multiple samples in academic research labs may necessitate a significant quantity of hand pipetting. This pipetting can discourage differences in the yield of target biomolecules between experiments and people. Staff and students may require extensive training and practice to produce constant protein yields. It is due to the numerous benefits of magnetic resins, such as their ease of use, rapid experimental protocols, suitability, and convenience for high-throughput automated and miniaturized processing.
Workflow
The separation of antibodies present in complex mixtures using chromatography involves the antibodies binding the beads. After Binding, the beads are washed to remove non-antibody protein. Finally, the antibody was eluted from the beads.
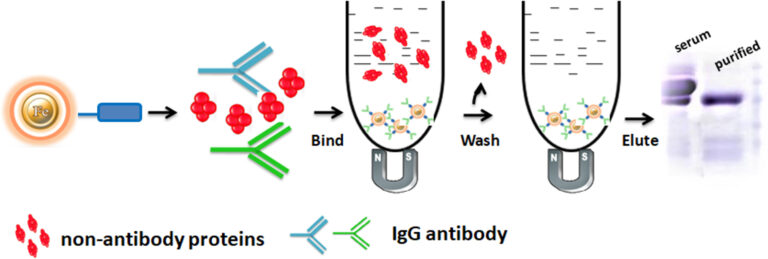
Feature and Benefits
●
Efficient – recovery exceeds 85%, and purity exceeds 85%.
●
Fast purification – Fast and high-throughput procedure
●
Convenient – no columns, filters, or a laborious repeat of pipetting or centrifugation.
●
Amine – free buffer does not require removal or neutralization
●
Robust – effective for IgG subclasses that bind poorly to Protein A or G
●
Gentle – No harsh antibody elution conditions helps retain IgG activity
●
Regenerable – beads can be renewed and used for up to 3 purifications
PROTOCOL
Note:
●
The following protocol is an example of purifying antibodies from the serum.
●
The beads and sample volume can be rational Scale-up (or down).
Equipment
Item
Magnetic Rack for centrifuge tube
** Based on sample volume, the user can choose one of the following magnetic Racks
Source
• BcMag™ Rack-2 for holding two individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-01)
• BcMag™ Rack-6 for holding six individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-02)
• BcMag™ Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Bioclone, Cat. No. MS-03)
• BcMag™ Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-04)
Item
BcMag™ 96-well Plate Magnetic Rack.
Source
• BcMa™ 96-well Plate Magnetic Rack (side-pull) compatible with 96-well PCR plate and 96-well microplate or other compatible Racks (Bioclone, Cat. No. MS-05)
Item
Adjustable Single and Multichannel Pipettes
Item
Centrifuge with Swinging Bucket
Addition items are required if using 96-well PCR plates / tubes
Vortex Mixer
** The user can also use other compatible vortex mixers. However, the Time and speed should be optimized, and the mixer should be: Orbit ≥1.5 mm-4 mm, Speed ≥ 2000 rpm
Eppendorf™ MixMate™
Eppendorf, Cat. No. 5353000529
Tube Holder PCR 96
Eppendorf, Cat. No. 022674005
Tube Holder 1.5/2.0 mL, for 24 × 1.5 mL or 2.0 mL
Eppendorf, Cat. No. 022674048
Smart Mixer, Multi Shaker
BenchTop Lab Systems, Cat. No. 5353000529
1.5/2.0 mL centrifuge tube
96-well PCR Plates or 8-Strip PCR Tubes
PCR plates/tubes
** IMPORTANT! If using other tubes or PCR plates, make sure that the well diameter at the bottom of the conical section of PCR Tubes or PCR plates has to be ≥2.5mm.
Addition items are required if using 96-well micro-plates
Fisher Scientific™ Microplate Advanced Vortex Mixers
Fisher, Cat. No. 02-216-101
OHAUS Microplate Vortex Mixers
OHAUS, Cat. No. 30392160
Vortex Mixer
** The user can also use other compatible vortex mixers. However, the Time and speed should be optimized, and the mixer should be: Orbit ≥1.5 mm-4 mm, Speed ≥ 800 rpm
Clear Flat-Bottom Non-Binding Assay Microplates
Items
Magnetic Rack for centrifuge tube
** Based on sample volume, the user can choose one of the following magnetic Racks
Source
●
BcMag™ Rack-2 for holding two individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-01)
●
BcMag™ Rack-6 for holding six individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-02)
●
BcMag™ Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Bioclone, Cat. No. MS-03)
●
BcMag™ Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-04)
BcMag™ 96-well Plate Magnetic Rack
●
BcMa™ 96-well Plate Magnetic Rack (side-pull) compatible with 96-well PCR plate and 96-well microplate or other compatible Racks (Bioclone, Cat. No. MS-05)
Adjustable Single and Multichannel Pipettes
Centrifuge with Swinging Bucket
Addition items are required if using 96-well PCR plates/tubes
Vortex Mixer
** The user can also use other compatible vortex mixers. However, the Time and Speed should be optimized, and the mixer should be: Orbit ≥1.5 mm-4 mm, Speed ≥ 2000 rpm
Eppendorf™ MixMate™
Tube Holder PCR 96
Tube Holder 1.5/2.0 mL, for 24 × 1.5 mL or 2.0 mL
Smart Mixer, Multi Shaker
Eppendorf, Cat. No. 5353000529
Eppendorf, Cat. No. 022674005
Eppendorf, Cat. No. 022674048
BenchTop Lab Systems, Cat. No. 5353000529
Eppendorf™ MixMate™
Tube Holder PCR 96
Tube Holder 1.5/2.0 mL, for 24 × 1.5 mL or 2.0 mL
Smart Mixer, Multi ShakerEppendorf, Cat. No. 5353000529
Eppendorf, Cat. No. 022674005
Eppendorf, Cat. No. 022674048BenchTop Lab Systems, Cat. No. 5353000529
1.5/2.0 mL centrifuge tube
96-well PCR Plates or 8-Strip PCR Tubes
PCR plates/tubes
! IMPORTANT ! If using other tubes or PCR plates, make sure that the well diameter at the bottom of the conical section of PCR Tubes or PCR plates has to be ≥2.5mm.
Addition items are required if using 96-well microplates
Fisher Scientific™ Microplate Advanced Vortex Mixers
Fisher, Cat. No. 02-216-101
OHAUS Microplate Vortex Mixers
OHAUS, Cat. No. 30392160
Vortex Mixer
** The user can also use other compatible vortex mixers. However, the Time and Speed should be optimized, and the mixer should be: Orbit ≥1.5 mm-4 mm, Speed ≥ 800 rpm
Clear Flat-bottom Non-Binding Assay Microplates
Procedure
! Important !
●
The following protocol is optimized for the efficient clean-up of the 3μl serum sample. The procedure may need to be optimized if an alternative reaction scale is used.
●
Shake or vortex the bottle to completely resuspend the magnetic beads before using.
●
Do not allow the magnetic beads to sit for more than two minutes before dispensing.
A.
Sample preparation
1.
Dilute whole serum with an equal volume of 2x Binding/Wash Buffer and mix well.
B.
Magnetic Particles Preparation
1.
Shake the bottle to completely resuspend the magnetic particles.
2.
Transfer desired amount of magnetic particles to a centrifuge tube (Note: 1 ml of BcMag™ Quick Antibody Purification Magnetic particles per 0.5-1 ml of whole serum is recommended. The volumes can be scaled accordingly. IgG levels in serum are usually 10-15mg/ml). Place the tube on the magnetic rack for 1-3 minutes until the supernatant becomes clear. Remove the supernatant while the tube remains on the rack.
3.
Remove the tube and resuspend the beads with 10 beads volumes of 1x Binding/Wash buffer.
C.
Sample Binding
1.
Add the washed particles from step B.4 to the diluted sample from step A1. Completely resuspend the beads and leave them at room temperature for 10-30 minutes with end-over-end rotation.
2.
Place the tube on a magnetic rack for 1-3 minutes. Remove the supernatant while the tube remains on the rack.
3.
Place the tube on the magnetic rack for 1-3 minutes. Remove the supernatant while the tube remains on the rack. Remove the tube from the rack and wash the particles with 10 beads volumes of 1x Binding/Wash Buffer.
4.
Repeat step 3 five times
D.
IgG Elution
1.
Remove the tube from the rack and resuspend the particles with 10 beads volumes of Elution buffer to elute IgG. Place the tube on the magnetic rack for 1-3 minutes. Remove the supernatant while the tube remains on the rack.
Mini-scale high throughput purification
1.
Transfer 50μl beads (Step B4) to a new well of 96well PCR plate or 96-well microplates or 0.2ml PCR tube and add 20μl diluted serum from A1.
2.
Mix the beads with the sample by slowly pipetting up and down 25 times (one minute) or by vortex mixer for 5 minutes at 2000 rpm.

3.
Place the sample plate/ tube on the magnetic separation plate for 30 seconds or until the solution is clear.
4.
Discard the supernatant and wash the beads three times with 100 μl of 1x Binding/Wash Buffer using a magnetic rack.
5.
Add 50 μl of Elution buffer to elute the antibody by slowly pipetting up and down 25 times (one minute) or vortex mixer for 5 minutes at 2000 rpm.
6.
Place the sample plate/ tube on the magnetic separation plate for 30 seconds or until the solution is clear.
7.
Transfer the supernatant to a clean plate /tube while the sample plate remains on the magnetic separation plate. The sample is ready for downstream applications.
Additional information
Beads Regeneration
1.
If the bead has to be regenerated, add 10ml of 35 mM sodium phosphate; 30% n-propanol, pH 8.5 for 1 ml beads and mix the beads with the sample by slowly pipetting up and down 25 times. Place the sample plate/ tube on the magnetic separation plate for 30 seconds or until the solution is clear. Discard the supernatants.
2.
Repeat Step 1 five times.
3.
Wash the beads with 10 beads volumes of 1x Binding/Wash Buffer three times
4.
Resuspend the beads in 1x Binding/Wash Buffer and store at 4°C. The beads may be renewed three times without losing substantial selectivity.
Troubleshooting
Problem
The yield of the purified antibody is too low or undetectable
Probable Cause
Sample devoid of antibody
Suggestion
- Ensure that the sample contains IgG by using another method, such as an ELISA or isotyping kit.
Problem
The yield of the purified antibody is too low or undetectable
Probable Cause
The antibody of interest bound to the beads
Suggestion
- Ensure that the pH of the sample is between 8.5.
Problem
Observe multiple bands present on stained SDS-polyacrylamide gel
Probable Cause
The ratio of the beads and sample is not optimized.
Suggestion
- Dialyze sample against 1x biding/wash buffer.
- Optimize the ration of the beads and sample
Problem
A significant amount of antibodies was purified. However, no antibody of interest was found.
Probable Cause
The concentration of antibodies of interest is low.
Suggestion
- Purify the antibody utilizing active affinity magnetic beads and a specific antigen.
Problem
Probable Cause
Suggestion
The yield of the purified antibody is too low or undetectable
Sample devoid of antibody
- Ensure that the sample contains IgG by using another method, such as an ELISA or isotyping kit.
The antibody of interest bound to the beads
- Ensure that the pH of the sample is around 8.5.
Observe multiple bands present on stained SDS-polyacrylamide gel
The ratio of the beads and sample is not optimized.
- Dialyze sample against 1x biding/wash buffer.
- Optimize the ration of the beads and sample
A significant amount of antibodies was purified. However, no antibody of interest was found.
The concentration of antibodies of interest is low.
- Purify the antibody utilizing active affinity magnetic beads and a specific antigen.
Learn More
Instruction Manual
MSDS
Related Antibody Magnetic Beads →General Reference
1.
Li M, Zhang Q, Lin D, Yao S. Development and application of hydrophobic charge-induction chromatography for bioseparation. J Chromatogr B Analyt Technol Biomed Life Sci. 2019 Dec 15;1134-1135:121850.
2.
Zhao G, Sun Y. Displacement chromatography of proteins on hydrophobic charge induction adsorbent column. J Chromatogr A. 2007 Sep 21;1165(1-2):109-15.
3.
Boschetti E. Antibody separation by hydrophobic charge induction chromatography. Trends Biotechnol. 2002 Aug;20(8):333-7.
4.
Gomes CS, Fashina A, Fernández-Castané A, Overton TW, Hobley TJ, Theodosiou E, Thomas OR. Magnetic hydrophobic-charge induction adsorbents for the recovery of immunoglobulins from antiserum feedstocks by high-gradient magnetic fishing. J Chem Technol Biotechnol. 2018 Jul;93(7):1901-1915.
5.
Hirano A, Maruyama T, Shiraki K, Arakawa T, Kameda T. A study of the small-molecule system used to investigate the effect of arginine on antibody elution in hydrophobic charge-induction chromatography. Protein Expr Purif. 2017 Jan;129:44-52.



