
BcMag™ Secreted His-tagged protein purification kit is based on magnetic beads coupled with a unique, proprietary ligand loaded with nickel ions. The ligands are extraordinarily firmly bonded and have a high affinity for His-tagged proteins, and exhibit low metal, ion-leaching properties even in the presence of chemical additives such as chelators (EDTA), strong reducing agents (DTT), or components of cell culture supernatants, which typically strip off Ni ions and reduce the functionality of most IMAC magnetic beads. The His-tagged protein purification resins allow the efficient purification of recombinant polyhistidine-tagged proteins directly from a soluble intracellular protein extract, HeLa, CHO mammalian cells, or Sf9 insect cells culture supernatant. They can be used manually with a magnetic stand or automatically with an instrument. It avoids extensive and time-consuming sample pretreatment processes, such as buffer exchange by dialysis in conjunction with concentration operations.
Magnetic resins have significant advantages over traditional chromatography such as columns, agarose, or non-magnetic resin. The magnetic bead-based format enables rapid high-yield processing of 96 samples in about 20 minutes, achieving more than 85% purities. When using column-based technologies, processing multiple samples in academic research labs may necessitate a significant quantity of hand pipetting. This pipetting can discourage differences in the yield of target biomolecules between experiments and people. Staff and students may require extensive training and practice to produce constant protein yields. It is due to the numerous benefits of magnetic resins, such as their ease of use, rapid experimental protocols, suitability, and convenience for high throughput automated and miniaturized processing.
Workflow
The workflow with magnetic resin is straightforward.
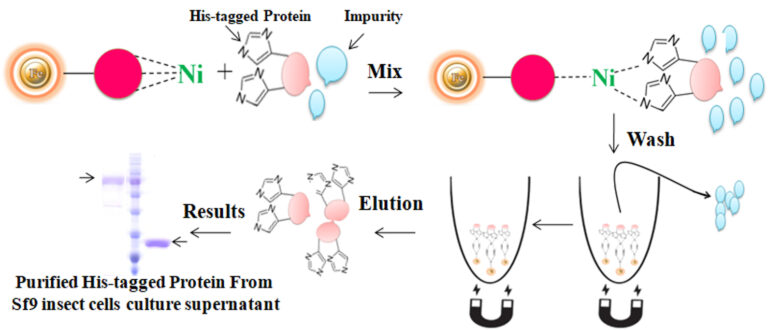
Mix the resins with the sample and incubate them with continuous rotation for a sufficient time. During mixing, the resins remain suspended in the sample solution, allowing the target molecules to interact with the immobilized ligand. After incubation, the beads are collected and separated from the sample using a magnet rack. Then the ultrapure His-tagged recombinant proteins are eluted by imidazole. During mixing, the beads remain suspended in the sample solution, allowing the target molecules to interact with the immobilized ligand. After incubation, the beads are collected and separated from the sample using a magnet rack. Then the ultrapure His-tagged recombinant proteins are eluted by imidazole.
Features and Advantages
●
Magnetic beads exhibit less nonspecific binding than porous supports.
●
Stable covalent bond with minimal ligand leakage
●
The beads resist up to 20 mM EDTA and 20 mM reducing reagents without nickel leaching.
●
Compatible with purification of overexpressed, secreted proteins in cell culture media.
●
High protein purity
●
Cost-effective: Eliminates columns, filters, repeat pipetting, and organic reagents.
●
High throughput: Compatible with many different automated liquid handling systems.
Applications
●
Investigating protein structure and function
●
Preparing recombinant proteins for X-ray crystallography
●
Ideal for the study of protein interactions with protein or DNA
●
Immunization to raise antibodies against a protein of interest.
●
Effective screening protein expression, even with crude cell lysates
●
Microscale purification of his-tagged proteins.
Background information
A polyhistidine tag called 6xHis-tag, His-tagged, and His-tag is a versatile tool for purifying the highly purified recombinant protein from various expression systems, including bacterial, yeast, plant cell, and mammalian cell systems. The tag comprises six or more consecutive histidine amino acid residues positioned at either N or C terminus of a recombinant protein. Due to its small size, His-tag has several distinctive features, including less immunogenicity, hydrophilic nature, and versatility under native and denaturing conditions.
Additionally, it is unnecessary to cleave the tag from the recombinant protein since it rarely perturbs the structure and function of its fusion protein. The purification principle of the His-tag depends on immobilized metal ion affinity chromatography (IMAC).
Immobilized metal ion affinity chromatography (IMAC) is a rapid affinity purification chromatography where the his-tagged protein are separated based on their affinity for Ni2+ or Co2+that have been immobilized by a chelator to a solid matrix such as beaded agarose or column. At pH 7-8, his tagged protein will bind to Ni2+ or Co2+. The binding reaction with the tagged protein is affected by various independent variables such as pH, temperature, salt type, concentration, immobilized metal and ligand density, and protein size. The bound protein is eluted by a decreasing pH gradient, increasing imidazole concentration, or adding an EDTA chelator in a buffer. This technique is an ideal tool for capturing and purification of his-tagged recombinant protein in a quick, inexpensive, and straightforward manner.Learn More
Instruction Manual
MSDS
Recombinant Protein & Purification Related Products →General Reference
1.
Carlsson M, Granér T, Algotsson M, Andersson LC, Björner M, Glad G, Hörnsten L, Le Greves P, Lindgren H, Lindqvist S, Öberg K, Hedlund H. New IMAC Media Enabling Purification of Histidine-tagged Proteins Directly From Eukaryotic Cell Culture Supernatants. J Biomol Tech. 2013 May;24(Suppl):S66.
2.
Spriestersbach A, Kubicek J, Schäfer F, Block H, Maertens B. Purification of His-Tagged Proteins. Methods Enzymol. 2015;559:1-15.
3.
Zhang C, Fredericks D, Longford D, Campi E, Sawford T, Hearn MT. Changed loading conditions and lysate composition improve the purity of tagged recombinant proteins with tacn-based IMAC adsorbents. Biotechnol J. 2015 Mar;10(3):480-9.
4.
Pina AS, et al. (2014) Affinity tags in protein purification and peptide enrichment: An overview. Methods in molecular biology (Clifton, N.J.) 1129: 147-168.
5.
Young CL, et al. (2012) Recombinant protein expression and purification: A comprehensive review of affinity tags and microbial applications. Biotechnol J 7(5): 620-634.



