
Hydroxyapatite, also known as hydroxylapatite (HA), is a calcium phosphate with morphology and composition like human hard tissues. It is a naturally occurring mineral with the chemical formula Ca5(PO4)3(OH)2. Hydroxyapatite can have a mixed charge on its surface, meaning that it has both positive and negative charges depending on the pH of the surrounding environment. At low pH values (acidic conditions), the surface of hydroxyapatite can become positively charged due to the protonation of some of its surface groups. Conversely, at high pH values (alkaline conditions), the surface of hydroxyapatite can become more negatively charged due to the deprotonation of its surface groups. Hydroxyapatite-based chromatography columns are commonly used in biotechnology and pharmaceutical industries for the purification of proteins and enzymes. They can also be used to purify DNA and RNA from complex mixtures.
BcMag™ Hydroxyapatite Magnetic Beads are magnetic particles that have a uniform coating of hydroxyapatite functional groups on their surface (as shown in Figure 1). These beads can be used to process 96 samples quickly and with high yields in just 20 minutes. They are capable of purifying antibodies, nucleic acids, viruses, and other large molecules from complex biological samples, either manually or with automated processes.
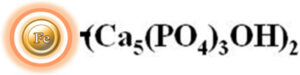
The hydroxyapatite magnetic beads replace traditional chromatographic matrices such as agarose, cellulose, sepharose, and Sephadex-based columns or resins for more efficiently processing. In column-based procedures, the lysate is centrifuged or cleared, the supernatant is added to the column, the membrane or resin is washed with buffer through centrifugation or vacuum manifold, and the required biomolecules are eluted in an adequate volume of buffer. When using column-based technologies, processing multiple samples in academic research labs may necessitate a significant quantity of hand pipetting. This pipetting can discourage differences in target biomolecule yield between experiments and people. Staff and students may require extensive training and practice to produce constant protein yields.
The hydroxyapatite resins have significant advantages over non-magnetic resin technologies. It is due to the numerous benefits of magnetic resins, such as their ease of use, rapid experimental protocols, suitability, and convenience for high-throughput automated and miniaturized processing. They thus see increasing use in various areas of life-sciences research and development, including drug discovery, biomedicine, bioassay development, diagnostics, genomics, and proteomics.
feature and benefits
●
Fast and straightforward – Magnetic beads-based format eliminates columns or filters or a laborious repeat of pipetting or centrifugation.
●
Convenient and expandable – Magnetic format enables high-throughput processing of multiple samples in parallel with many different automated liquid handling systems
●
Robust – Magnetic resins do not crack or run dry.
●
Low bed volume – Working with small magnetic bead volumes allows for minimal buffer volumes, resulting in concentrated elution fractions.
●
Chemical and thermal stability – A wide range of chemical compatibilities (aqueous and inorganic solvents), heat stability (autoclavable), and pH tolerance (pH >5.5) let hydroxyapatite be utilized below settings that improve nucleic acid and protein binding.
Learn More
Instruction Manual
MSDS
Related Ion Exchange Magnetic Beads →General Reference
1.
Gagnon P. Monoclonal antibody purification with hydroxyapatite. N Biotechnol. 2009 Jun;25(5):287-93.
2.
Gagnon P, Beam K. Antibody aggregate removal by hydroxyapatite chromatography. Curr Pharm Biotechnol. 2009 Jun;10(4):440-6.
3.
Wang Y, Carta G. Competitive binding of monoclonal antibody monomer-dimer mixtures on ceramic hydroxyapatite. J Chromatogr A. 2019 Feb 22;1587:136-145.
4.
Cummings LJ, Frost RG, Snyder MA. Monoclonal antibody purification by ceramic hydroxyapatite chromatography. Methods Mol Biol. 2014;1131:241-51.
5.
Cummings LJ, Snyder MA, Brisack K. Protein chromatography on hydroxyapatite columns. Methods Enzymol. 2009;463:387-404.
6.
Hilbrig F, Freitag R. Isolation and purification of recombinant proteins, antibodies and plasmid DNA with hydroxyapatite chromatography. Biotechnol J. 2012 Jan;7(1):90-102.
7.
Broadhurst AV. Hydroxylapatite chromatography. Curr Protoc Protein Sci. 2001 May;Chapter 8:Unit8.6.
8.
Niimi M, Masuda T, Kaihatsu K, Kato N, Nakamura S, Nakaya T, Arai F. Virus purification and enrichment by hydroxyapatite chromatography on a chip. Sens Actuators B Chem. 2014 Oct 1;201:185-190.
9.
Duffy E, Florek J, Colon S, Gerdon AE. Selected DNA aptamers as hydroxyapatite affinity reagents. Anal Chim Acta. 2020 May 8;1110:115-121.
10.
Brundin M, Figdor D, Sundqvist G, Sjögren U. DNA binding to hydroxyapatite: a potential mechanism for preservation of microbial DNA. J Endod. 2013 Feb;39(2):211-6.



