
Magnetic spheres segregate proteins or nucleic acids with the use of beads-adsorbent technique as a chromatographic matrix. Ion exchange chromatography is extensively employed to segregate or refine a targeted molecule from raw biological materials. The molecules are discriminated based on differences in their available surface charges utilizing extremely gentle binding and eluting conditions to preserve their biological activity intact.
BcMag™ DEAE Magnetic Beads are uniform magnetic resins grafted with a high density of diethyl aminoethyl (DEAE) functional groups on the surface (Fig.1). The weak anion exchange magnetic bead-based format enables rapid high-yield processing of 96 samples in about 20 minutes. It can quickly fraction proteins or nucleic acids from complex biological samples (such as serum, plasma, etc.) manually or automatically. The purified protein can be used in downstream applications such as sample fractionation for 1D and 2D SDS-PAGE, X-ray crystallization, and NMR spectroscopy. The Weak Ion Exchange resins allow the rapid release of very strong ions that may be retained irreversibly on Strong Ion Exchange beads. Additionally, weak ion exchangers can be effective separation tools when strong ion exchangers fail because the selectivity of weak and strong ion exchangers frequently differ.
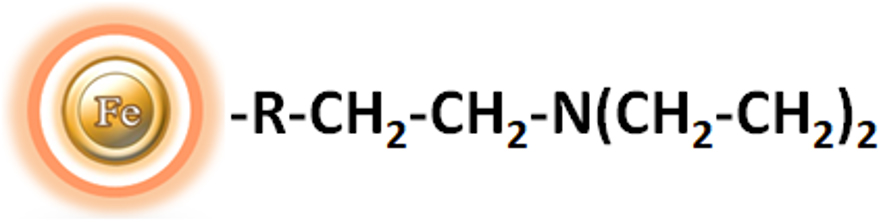
Weak anion exchange magnetic resins are used to replace time-consuming, complex, and costly chromatographic procedures such as agarose, cellulose, Sepharose, and Sephadex-based columns or resins. In column-based procedures, the lysate is centrifuged or cleared, the supernatant is added to the column, the membrane or resin is washed with buffer through centrifugation or vacuum manifold, and the required biomolecules are eluted in an adequate volume of buffer. When using column-based technologies, processing multiple samples in academic research labs may necessitate a significant quantity of hand pipetting. This pipetting can discourage differences in target biomolecule yield between experiments and people. Staff and students may require extensive training and practice to produce constant protein yields.
Weak anion exchange magnetic resins have significant advantages over non-magnetic resin technologies. It is due to the numerous benefits of magnetic resins, such as their ease of use, rapid experimental protocols, suitability, and convenience for high throughput automated and miniaturized processing. They thus see increasing use in various areas of life-sciences research and development, including drug discovery, biomedicine, bioassay development, diagnostics, genomics, and proteomics.
Weak anion exchange beads feature and benefits
●
Fast and simple – DEAE magnetic beads-based format eliminates columns or filters or a laborious repeat of pipetting or centrifugation.
●
Convenient and expandable – Magnetic format enables high-throughput processing of multiple samples in parallel with many different automated liquid handling systems.
●
Robust – DEAE Magnetic beads do not crack or run dry.
●
Low bed volume – Working with small magnetic bead volumes allows for minimal buffer volumes, resulting in concentrated elution fractions.
DEAE magnetic beads Applications
●
Protein pre-fractionation in cell lysates
●
Optimizing purification conditions for new protein preparation protocols
●
Protein purification and concentration
●
Antibody purification from serum, ascites, or tissue culture supernatant
●
Preparation of samples before 1D or 2D PAGE
●
Phosphopeptide purification before MS analysis
Learn More
Instruction Manual
MSDS
Related Ion Exchange Magnetic Beads →General Reference
1.
Dasgupta PK, Maleki F. Ion exchange membranes in ion chromatography and related applications. Talanta. 2019 Nov 1;204:89-137
2.
Wittkopp F, Peek L, Hafner M, Frech C. Modeling and simulation of protein elution in linear pH and salt gradients on weak, strong and mixed cation exchange resins applying an extended Donnan ion exchange model. J Chromatogr A. 2018 Apr 13;1545:32-47.
3.
Staby A, Jensen RH, Bensch M, Hubbuch J, Dünweber DL, Krarup J, Nielsen J, Lund M, Kidal S, Hansen TB, Jensen IH. Comparison of chromatographic ion-exchange resins VI. Weak anion-exchange resins. J Chromatogr A. 2007 Sep 14;1164(1-2):82-94.
4.
Staby A, Jacobsen JH, Hansen RG, Bruus UK, Jensen IH. Comparison of chromatographic ion-exchange resins V. Strong and weak cation-exchange resins. J Chromatogr A. 2006 Jun 23;1118(2):168-79.
5.
Fishman JB, Berg EA. Purification of Antibodies: diethylaminoethyl (DEAE) Chromatography. Cold Spring Harb Protoc. 2019 Jan 2;2019(1).
6.
Černigoj U, Vidič J, Ferjančič A, Sinur U, Božič K, Mencin N, Martinčič Celjar A, Gagnon P, Štrancar A. Guanidine improves DEAE anion exchange-based analytical separation of plasmid DNA. Electrophoresis. 2021 Dec;42(24):2619-2625.
7.
Shields PA, Farrah SR. Characterization of virus adsorption by using DEAE-sepharose and octyl-sepharose. Appl Environ Microbiol. 2002 Aug;68(8):3965-8.



