
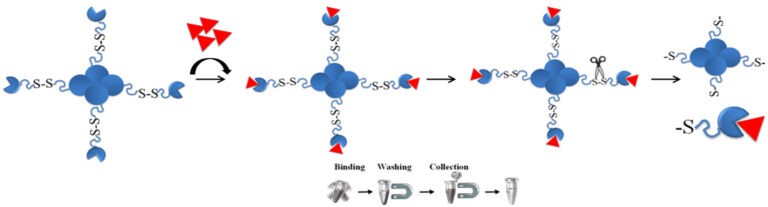
BcMag™ Cleavable Streptavidin Magnetic Beads are uniform superparamagnetic microspheres coated with high purity of (>97%) streptavidin. Since the streptavidin is linked to the beads (particles) through a built-in cleavable disulfide linker, the reducing agents such as DTT or β-mercaptoethanol can cleave and separate the biotinylated molecule-streptavidin complex from the beads instead of harsh elution reagents such as 8M guanidine or SDS detergent after affinity purification. The microspheres are specifically designed and tested for applications in immunoprecipitation, cell sorting, and rapid single-step capture of biotinylated molecules such as DNA, RNA, antibody, or protein from the cell.
The interaction between streptavidin (or avidin n) and biotin exhibits one of the highest known non-covalent interactions). Avidin, streptavidin, monomeric avidin, and their analogs have become powerful tools for probes and affinity ligands for various applications in biochemical assays, diagnosis, affinity purification, and drug delivery. Streptavidin is a tetrameric biotin-binding protein that originates from Streptomyces and has a mass of 60,000 daltons. Streptavidin has no carbohydrate and lower pI, giving a lower degree of nonspecific binding, making streptavidin an ideal reagent choice for many detection systems.
Features and Advantages
Advantages and benefits of using streptavidin biotin-binding systems
●
High affinity
Streptavidin is homo-tetramer and has a higher high biotin-binding affinity, with a KD ∼10−14 M.●
High stability
The biotin-binding complex is exceptional stability and can resist Extreme pH, temperature (2 °C and 40 °C), harsh organic solvents, denaturing agents such as guanidinium chloride, detergents such as SDS and proteolytic enzymes.
●
Outstanding selectivity and specificity
The interaction of the biotin and streptavidin is highly specific, ensuring low nonspecific binding.
●
High sensitivity
High stability and specificity ensure high detection sensitivity.
●
Very flexibility
The small size and remarkable stability of biotin are proven to be exceptionally suitable for relatively easy incorporation into various molecules such as synthetic polymers, fluorophores, small molecules, or into specific locations in biomolecules that impose minimal perturbation to the structure and function of the conjugated biomolecules.
Advantages and benefits of using streptavidin magnetic microspheres
●
Easily release the biotinylated molecule-streptavidin complex from the particles
●
Quick, Easy, and one-step high-throughput procedure: eliminates Columns or filters or a laborious repeat of pipetting or centrifugation.
●
High binding capacity
●
Exhibits low nonspecific binding
●
Scalable – easily adjusts for sample size and automation
Protocol
Materials Required
●
Binding Buffer: 1x PBS, 0.1% BSA, pH 7.4
●
Magnetic Rack (for manual operation)
Based on sample volume, the user can choose one of the following Magnetic Racks:
– BcMag™ Magnetic Rack-2 for holding two individual 1.5 ml centrifuge tubes (Cat. No. MS-01);– BcMag™ Magnetic Rack-6 for holding six individual 1.5 ml centrifuge tubes (Cat. No. MS-02);
– BcMag™ Magnetic Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Cat. No. MS-03);
– BcMag™ Magnetic Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Cat. No. MS-04);
– BcMag™ Magnetic Rack-96 for holding a 96 ELISA plate or PCR plate (Cat. No. MS-05).
Procedure
Note:
●
This protocol is a general affinity purification procedure. To obtain the best results, each user should determine the optimal working conditions for the purification of the individual target protein.
●
We strongly recommended titration to optimize the number of particles used for each application based on the amount of the target biotinylated molecules in the crude sample based on the particles binding capacity. Too many magnetic particles used will cause higher backgrounds, while too few particles used will cause lower yields.
1.
Transfer the optimal amount of the beads to a centrifuge tube. Place the tube on the magnetic rack for 1-3 minutes. Remove the supernatant while the tube remains on the rack.
2.
Remove the tube and wash the beads with 5-bed volumes of PBS buffer by vortex for 30 seconds. Leave the tube at room temperature for 1-3 minutes. Place the tube on the magnetic rack for 1-3 minutes. Remove the supernatant while the tube remains on the rack.
3.
Repeat step 2 two times.
4.
Add washed beads to the crude sample containing target molecules and incubate at room temperature or desired temperature for 1-2 hours (Lower temperature require longer incubation time).
Note: Strongly recommended to perform a titration to optimize incubation time. More prolonged incubation may cause higher background.
5.
Extensively wash the beads with 5-beads volumes of PBS buffer or 1M NaCl until the absorbance of eluting at 280 nm approaches the background level (OD 280 < 0.05).Note: Adding a higher concentration of salts, nonionic detergent, and reducing agents may reduce the nonspecific background. For example, the addition of NaCl (up to 1-1.5 M), 0.1-0.5% nonionic detergents such as Triton X 100 or Tween 20 to the washing buffer.
6.
Cleave the Disulfide Bond
Note: Due to conformational variation from ligands to ligands, the user should determine the optimal working conditions such as reducing agent, pH, and temperature for cleaving the disulfide bond of individual ligands. The following is an example of cleaving conjugated GFP from the beads.
1)
Incubate the magnetic beads (30mg/ml) in either 140 mM β-mercaptoethanol or 5mM DTT (Dithiothreitol).
a. 100 mM Tris-HCl, pH 8.0, 50 mM EDTA, 140 mM β-mercaptoethanol for 2 hours to overnight at room temperature or 98°C for 5 minutes.
b. 100 mM Tris-HCl, pH 8.0, 50 mM EDTA, 5mM DTT for 2 hours to overnight at room temperature or 98°C for 5 minutes.
Learn More
Instruction Manual
MSDS
Related Affinity Magnetic Beads →General Reference
1.
Chivers CE, Koner AL, Lowe ED, Howarth M. How the biotin-streptavidin interaction was made even stronger: investigation via crystallography and a chimeric tetramer. Biochem J. 2011;435(1):55-63. doi:10.1042/BJ20101593
2.
Dundas CM, Demonte D, Park S. Streptavidin-biotin technology: improvements and innovations in chemical and biological applications. Appl Microbiol Biotechnol. 2013 Nov;97(21):9343-53. doi: 10.1007/s00253-013-5232-z. Epub 2013 Sep 22. PMID: 24057405.
3.
Lakshmipriya T, Gopinath SC, Tang TH. Biotin-Streptavidin Competition Mediates Sensitive Detection of Biomolecules in Enzyme Linked Immunosorbent Assay. PLoS One. 2016 Mar 8;11(3):e0151153. doi: 10.1371/journal.pone.0151153. PMID: 26954237; PMCID: PMC4783082.
4.
Yang H, Zhang Q, Liu X, Yang Y, Yang Y, Liu M, Li P, Zhou Y. Antibody-biotin-streptavidin-horseradish peroxidase (HRP) sensor for rapid and ultra-sensitive detection of fumonisins. Food Chem. 2020 Jun 30;316:126356. doi: 10.1016/j.foodchem.2020.126356. Epub 2020 Feb 4. PMID: 32045810.



