
The activated magnetic beads are uniform inert silica-based magnetic beads grafted with a high density of alkyne functional groups on the surface. The beads are specifically designed to efficiently enrich azide-tagged biomolecules from complex cell lysates via a Cu(I)-catalyzed Alkyne-Azide (CUAAC) reaction. Compared with other affinity resins such as agarose beads or other polymers, the inert silica enclosed magnetic beads offer high stability, low nonspecific binding, and superior handling in protein-based systems. Since the active cleavable alkyne group is linked with the beads through a built-in cleavable disulfide linker, reducing agents such as DTT or β-mercaptoethanol can cleave and separate the target molecule-ligand complex from the beads after affinity purification. These magnetic beads are ideal tools for genomics, proteomics, biomarker discovery, posttranslational modification (PTM) analysis, etc.
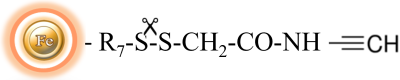
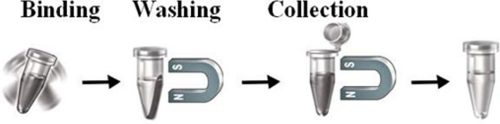
Workflow
The beads work perfectly as affinity resin for capturing azide-tagged biomolecules from complex cell lysate. Add the beads to a sample containing the tagged biomolecules, then mix, incubate, wash and elute the target molecules.
Features and Advantages
●
Easy to use
●
Stable covalent bond with minimal ligand leakage
●
Produces reusable immunoaffinity matrices
●
Low nonspecific binding
1.
Cleave the Disulfide Bond
Note: Due to conformational variation from ligands to ligands, the user should determine the optimal working conditions such as reducing agent, pH, and temperature for cleaving the disulfide bond of individual ligands.
1)
Incubate the magnetic beads (30mg/ml) in either 140 mM β-mercaptoethanol or 5mM DTT (Dithiothreitol).
a. 100 mM Tris-HCl, pH 8.0, 50 mM EDTA, 140 mM β-mercaptoethanol for 2 hours to overnight at room temperature or 98°C for 5 minutes.
b. 100 mM Tris-HCl, pH 8.0, 50 mM EDTA, 5mM DTT for 2 hours to overnight at room temperature or 98°C for 5 minutes.
Learn More
Instruction Manual
MSDS
Related Affinity Magnetic Beads →


