
It is frequently helpful to immobilize affinity ligands via functional groups other than amines. The thiol group, in particular, can be employed to guide coupling processes away from active centers or binding sites on specific protein molecules.
Cysteines are frequently bonded between their side chains via disulfide bonds (-S-S-) as part of a protein’s secondary or tertiary structure. The side chain of cysteine contains sulfhydryls (-SH) (Cys, C). These must be converted to sulfhydryls before being immobilized. Thiol groups (sulfhydryls) can be found naturally in proteins or introduced through the reduction of disulfides or using various thiolation reagents.
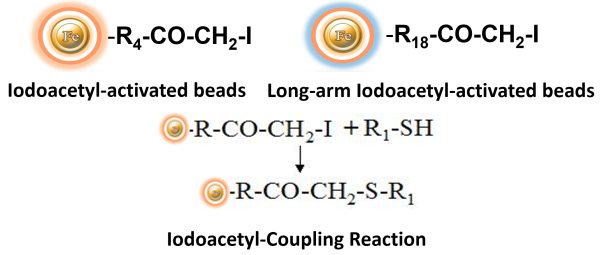
BcMag™ Iodoacetyl-activated Magnetic Beads are uniform, silica-based superparamagnetic beads coated with a high density of Iodoacetyl functional groups on the surface. It is designed to enable fast, efficient, and covalent immobilization of protein, peptides, and other ligands through their sulfhydryl groups (-SH) for affinity purification procedures. At physiological to alkaline circumstances (pH 7.2 to 9) ) in either aqueous or organic solvents with 20- 30% DMSO or DMF, iodoacetyl-activated supports react with sulfhydryl groups, resulting in stable thioether bonds. These reactions are often carried out in the dark to prevent the formation of free iodine, which can react with tyrosine, histidine, and tryptophan residues. The hydrophilic surface ensures beads low nonspecific adsorption, excellent dispersion, and easy handling in various buffers. BcMag™ Iodoacetyl-activated Magnetic Beads are most suitable for conjugation of a larger protein. BcMagTM Long-arm Iodoacetyl-activated Magnetic Beads are recommended to conjugate small peptides because the long-arm (20-atom) hydrophilic linker may reduce steric hindrance.
Workflow
The Iodoacetyl magnetic beads work perfectly as solid support for various bioseparations to refine molecules, cells, and parts of cells into purified fractions. After conjugation with ligands, add the beads to a solution containing the target molecules, then mix, incubate, wash and elute the target molecules.
Features and Benefits
●
Iodoacetyl groups react selectively with sulfhydryl (-SH) groups to produce irreversible thioether linkages.
●
Fast – couple peptide samples in 2 hours.
●
Versatile coupling conditions—as needed for protein or peptide solubility during the coupling reaction, employ pH 7.5 to 9.0 in aqueous buffers, organic solvent (e.g., 20% DMSO), or denaturant (guanidine HCl).
●
Simple to follow protocols for sample preparation, immobilization, and affinity purification
●
High capacity—Immobilize 15 -20 μg antibody/mg beads.
Applications
●
Immobilize peptides with terminal cysteine residues to purify antibodies raised against peptide immunogens.
●
Immobilize antibodies in an orientated manner using hinge-region sulfhydryls to ensure that antigen binding sites are not sterically inhibited when antigen affinity purification is performed.
●
Produces reusable immunoaffinity matrices
●
Maintains antibody function – immobilizes IgG via the Fc region, leaving both antigen binding sites available for target capture.
Learn More
Instruction Manual
MSDS
Related Affinity Magnetic Beads →


