
In their natural condition, most biological compounds do not contain carbonyl ketones or aldehydes. However, similar groups on proteins may be effective for forming an immobilization site that drives covalent coupling away from active centers or binding regions. Glycoconjugates, such as glycoproteins and glycolipids, typically contain sugar residues with hydroxyls on neighboring carbon atoms; these cis-diols can be oxidized with sodium periodate to produce aldehydes as covalent immobilization sites.
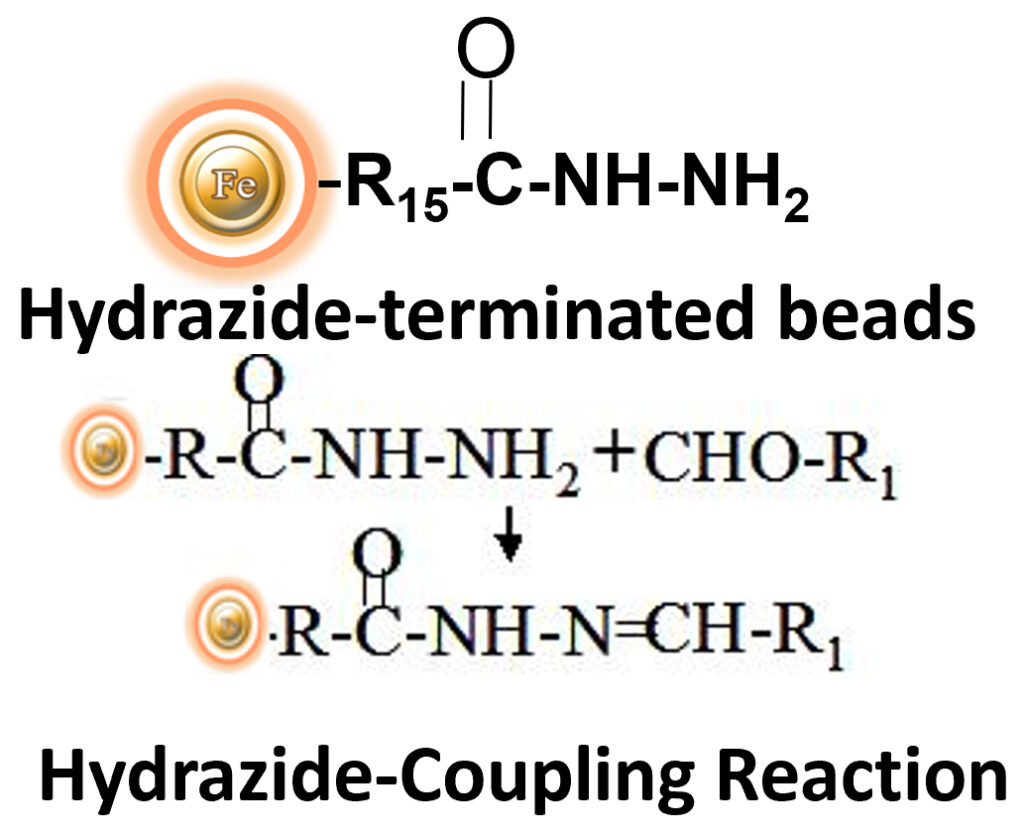
BcMag™ Glycoprotein & Antibody Conjugation Kit produces affinity support with glycoproteins and antibodies, which often have numerous carbohydrates (glycosylation) on their Fc sections, using hydrazide-activated magnetic beads and a specific catalyst. These kits may immobilize glycosylated proteins (including monoclonal antibodies) with periodate-oxidizable carbohydrate groups to create reusable affinity matrices. This hydrazide immobilization chemistry results in antibodies with unobstructed antigen-binding sites and excellent purification capacity. In a simple non-amine buffer containing the catalyst, the full coupling reaction takes 2 to 4 hours. Coupling efficiency with antibodies and common glycoproteins is typically greater than 85%, yielding 15μg to 20 μg of immobilized protein per mg of the hydrazide-terminated magnetic beads. The matrix prepared with stable glycoproteins or antibodies can be regenerated and reused at least five times without appreciable loss of binding capacity.BcMag™ Hydrazide-Terminated Magnetic Beads are uniform magnetic beads grafted with a high density of hydrazide functional groups on the surface. Hydrazide chemistry is effective for labeling, immobilizing, or conjugating glycoproteins via glycosylation sites, which are frequently (as with most polyclonal antibodies) positioned in domains distant from the critical binding sites whose function needs to be preserved. Coupling antibodies in this manner selectively targets heavy chains in the Fc portion of the molecule, assisting in the best possible preservation of antigen binding activity by the ends of the Fv regions. At pH 5 to 7, hydrazide-terminated supports and compounds will conjugate with carbonyls of oxidized carbohydrates (sugars), producing hydrazone linkages. The hydrazide beads are suitable for conjugating larger glycoproteins and glycolipids, carbohydrates or other ligands. Moreover, the hydrophilic surface ensures low nonspecific adsorption, excellent dispersion, and easy handling in various buffers.
Workflow
The Beads work perfectly as affinity resin for affinity purification to refine molecules, cells, and parts of cells into purified fractions. After conjugation with ligands, add the beads to a sample containing the target molecules, then mix, incubate, wash and elute the target molecules
Features and benefits
●
Specific immobilization—the hydrazide-activated beads bind exclusively to pure glycoproteins containing sugar groups that have been gently oxidized with periodate (e.g., sialic acid).
●
Stable covalent bond with low levels of ligand leakage
●
Maintains antibody function – Immobilizes IgG via the Fc region, leaving both antigen binding sites available for target capture.
●
Low nonspecific binding
●
High capacity – Immobilize 15-20μg antibody/mg beads
PROTOCOL
Note:
●
This protocol can be scaled up as needed. We strongly recommended titration to optimize the number of beads used for each application.
●
Avoid tris or other buffers containing primary amines because these will compete with the intended coupling reaction.
Materials Required
1.
Magnetic Rack (for manual operation)
Based on sample volume, the user can choose one of the following Magnetic Racks:
– BcMag™ Magnetic Rack-2 for holding two individual 1.5 ml centrifuge tubes (Cat. No. MS-01);
– BcMag™ Magnetic Rack-6 for holding six individual 1.5 ml centrifuge tubes (Cat. No. MS-02);
– BcMag™ Magnetic Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Cat. No. MS-03);
– BcMag™ Magnetic Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Cat. No. MS-04);
– BcMag™ Magnetic Rack-96 for holding a 96 ELISA plate or PCR plate (Cat. No. MS-05).
2.
Coupling Buffer: 0.1 M sodium phosphate, pH 7.0
3.
Oxidizing Agent: Sodium meta-Periodate (NaIO4) (Sigma, Cat. No. S1878)4.
Washing Buffer: 1M NaCl
5.
PBS Buffer
6.
2.5 μm BcMag™ Hydrazide-Terminated Magnetic Beads
Ligand Coupling
A.
Beads Preparation
1.
Weight 30 mg beads and add them into a 1.5 ml centrifuge tube.
2.
Add 1 ml coupling buffer and resuspend the beads very well by vortexing or pipetting.
3.
Insert the tube into a magnetic rack for 1-3 minutes until the supernatant becomes clear. Aspirate and discard the supernatant with a pipette while the tube remains in the rack.
4.
The beads are ready for coupling.
B.
Oxidation of Glycoprotein or other ligands
Note: The reaction is light sensitive and should be performed in the dark.
1.
Dissolve or dilute 0.5-10 mg glycoprotein or other ligands in 1 ml coupling buffer. (Note: If the protein or other ligands is already suspended in other buffers, perform a buffer exchange by dialysis or a desalting column.)
2.
Add the protein or other ligands solution to an amber vial containing 2 mg sodium meta-periodate (final concentration10mM). Swirl gently to dissolve the oxidizing agent.
3.
Incubate the sample in the dark at room temperature for 45 minutes with good mixing (end-over-end).
C.
Conjugation
1.
Add the oxidized protein or other ligands solution to the prepared magnetic beads from Step A4 and mix well by vortexing or pipetting.
Note: Coupling efficiency depends on the structure and the size of the target glycoprotein or other ligands. The user should empirically optimize the ratio of the protein to the beads.
2.
Incubate the reaction in the dark at room temperature overnight with good mixing (end-over-end).
3.
Insert the tube into a magnetic rack for 1-3 minutes until the supernatant becomes clear. Aspirate and discard the supernatant with a pipette while the tube remains in the rack. Remove the tube from the rack and resuspend the beads with 5 ml Coupling Buffer by vortex or pipette.
4.
Repeat steps 3 for three times.
5.
Resuspend the beads in PBS buffer with 0.01% azide (w/v) to desired concentration and store at 4°C until use. Do not freeze.
D.
General affinity purification Protocol
Note:
●
This protocol is a general affinity purification procedure. Designing a universal protocol for all protein purification is impossible because no two proteins are precisely alike. The user should determine the optimal working conditions for purifying the individual target protein to obtain the best results.
●
We strongly recommended titration to optimize the number of beads used for each application based on the amount of the target protein in the crude sample. Too many magnetic beads used will cause higher backgrounds, while too few beads used will cause lower yields. Each mg of magnetic beads typically binds to 10-20 μg of the target protein.
1.
Transfer the optimal amount of the beads to a centrifuge tube. Place the tube on the magnetic rack for 1-3 minutes. Remove the supernatant while the tube remains on the rack.
2.
Remove the tube and wash the beads with 5-bed volumes of PBS buffer by vortex for 30 seconds. Leave the tube at room temperature for 1-3 minutes. Place the tube on the magnetic rack for 1-3 minutes. Remove the supernatant while the tube remains on the rack.
3.
Repeat step 2 two times.
4.
Add washed beads to the crude sample containing the target protein and incubate at room or desired temperature for 1-2 hours (Lower temperatures require longer incubation time).
Note: Strongly recommended to perform a titration to optimize incubation time. More prolonged incubation may cause higher background.
5.
Extensively wash the beads with 5-beads volumes of PBS buffer or 1M NaCl until the absorbance of eluting at 280 nm approaches the background level (OD 280 < 0.05).
Note: Adding a higher concentration of salts, nonionic detergent, and reducing agents may reduce the nonspecific background. For example, adding NaCl (up to 1-1.5 M), 0.1-0.5% nonionic detergents such as Triton X 100 or Tween 20 to the washing buffer.
6.
Elute the target protein by appropriate methods such as low pH (2-4), high pH (10-12), high salt, high temperature, affinity elution, or boiling in an SDS-PAGE sample buffer.
7.
Transfer the optimal amount of the beads to a centrifuge tube. Place the tube on the magnetic rack for 1-3 minutes. Remove the supernatant while the tube remains on the rack.
8.
Remove the tube and wash the beads with 5-bed volumes of PBS buffer by vortex for 30 seconds. Leave the tube at room temperature for 1-3 minutes. Place the tube on the magnetic rack for 1-3 minutes. Remove the supernatant while the tube remains on the rack.
9.
Repeat step 2 two times.
10.
Add washed beads to the crude sample containing target protein and incubate at room temperature or desired temperature for 1-2 hours (Lower temperature require longer incubation time).
Note: Strongly recommended to perform a titration to optimize incubation time. More prolonged incubation may cause higher background.
11.
Extensively wash the beads with 5-beads volumes of PBS buffer or 1M NaCl until the absorbance of eluting at 280 nm approaches background level (OD 280 < 0.05).
Note: Adding a higher concentration of salts, nonionic detergent, and reducing agents may reduce the nonspecific background. For example, the addition of NaCl (up to 1-1.5 M), 0.1-0.5% nonionic detergents such as Triton X100 or Tween20 to the washing buffer.
12.
Elute the target protein by appropriate methods such as low pH (2-4), high pH (10-12), high salt, high temperature, affinity elution, or boiling in SDS-PAGE sample buffer.
Learn More
Instruction Manual
MSDS
Related Antibody Magnetic Beads →


