
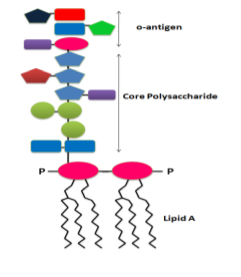
Endotoxins, also known as Lipopolysaccharides (LPS), are a type of pyrogen with large complex molecules. They consist of an innermost core of hydrophobic fatty acid groups and a central and outermost region composed of hydrophilic polysaccharides (Fig.1). They are part of the outer membrane of the cell wall of gram-negative bacteria pathogens (such as Escherichia coli, Salmonella, Shigella, Pseudomonas, Neisseria, Haemophilus influenzae, Bordetella pertussis, and Vibrio cholera ). They release into the circulation upon disrupting the intact bacteria (death, cell lysis).
The endotoxins are major contaminants found in commercially available biological products, which often adversely affect the study of the biological effects of the main ingredient. Gram-negative bacteria (e.g., Escherichia coli) are widely used in the biotechnology industry to produce recombinant products such as proteins, plasmid DNAs, and vaccines. These products can be contaminated with endotoxins at any point within the process. Removing Endotoxin from the product is critical since it can result in multiple pathophysiological effects, such as fever, shaking chills, septic shock, toxic pneumonitis, and respiratory symptoms lethality.
Removing endotoxin is one of the most challenging downstream processes during protein or DNA purification. Several methods are used to reduce endotoxin contamination of biological sample preparations, including affinity chromatography, such as immobilized polymyxin B, L-histidine, and poly-L-lysine, anion-exchange chromatography, gel filtration, ultrafiltration, sucrose gradient centrifugation, and Triton X-114 phase separation. The success of these techniques in reducing endotoxin contamination from a biological sample is strongly dependent on the properties of the target molecules. For example, ultrafiltration and ion exchange chromatography are commonly used techniques for removing endotoxin contaminants. Although ultrafiltration effectively removes endotoxins from water, it is not suitable for protein solution since the physical forces can damage the protein. Anion exchangers can effectively remove the Endotoxin but cause a significant loss of biological material due to adsorption. Many commercially available products are made from traditional chromatography matrices such as agarose resin or column. These solid matrices make the endotoxin removal process tedious, time-consuming, unable to handle very tiny samples, and challenging to adapt to the automation system. Bioclone introduces a powerful magnetic beads-based endotoxin removal system to overcome these problems.
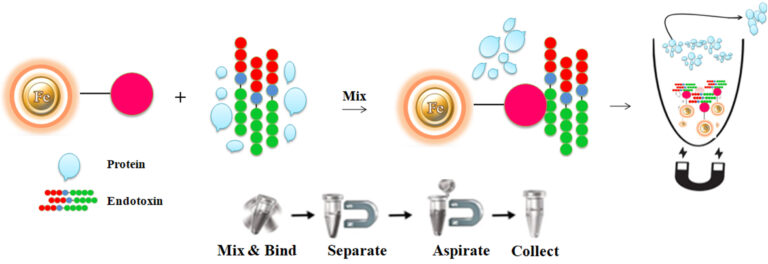
BcMag™ Quick Endotoxin Removal Kit uses magnetic microsphere covalently immobilized with a high density of polymyxin B to remove endotoxin. It is specially designed for quick Endotoxin removal from various sample types. Polymyxin B, a peptide antibiotic, has a very high binding affinity for the lipid A moiety of most endotoxins. The microspheres combine all the advantages of affinity protein purification (low costs, simplicity, high specificity, and capacity) and magnetic properties to perform efficient manual or automatic quick high-throughput Endotoxin removal.
Workflow
The purification with magnetic microparticles is straightforward.
1.
Mix the microparticles with the sample and incubate them with continuous rotation for a sufficient time. During mixing, the beads remain suspended in the sample solution, allowing the endotoxins to bind to the immobilized polymyxin B.
2.
After incubation, the beads are collected and separated from the sample using a magnet rack. Transfer the endotoxin-free supernatant to a fresh tube.
Learn More
Instruction Manual
MSDS
Sample Preparation Related Products →General Reference
1.
Caroff, M., Kariban, D., Cavaillon, J. et al. (2002). Structural and functional analyses of bacterial lipopolysaccharides. Microbes and Infection 4: 915-926
2.
Hecker, W., Witthauser, D. & Staerk, A. (1994). Validation of dry heat inactivation of bacteria endotoxins. PDA Journal of Pharmaceutical Science and Technology 48 (4): 197-204
3.
Sandle, T. (2004). Three aspects of LAL testing: Glucans, depyrogenation and water system qualification. PharMIG News 16: 3-12
4.
Williams, K.L. (2001). Endotoxins: Pyrogens, LAL testing and Depyrogenation 2nd Edition. Drugs and the Pharmaceutical Sciences Volume 111. Marcel Dekker Inc., New York, USA. Chapters 1, 2, 7 & 8



