
Human serum or plasma contains a wealth of proteomic information and is frequently analyzed for protein biomarkers of normal and pathological states. The extensive concentration range of proteins contained in human serum is one of the most challenging obstacles in studying it. Proteins that are abundantly expressed, such as albumin and IgG, account for 70 percent of total protein in serum/plasma. In contrast, protein biomarkers may present considerably lower amounts (pg/ml). High abundance proteins pose problems for analytical procedures like one-dimensional and two-dimensional electrophoresis, high-performance liquid chromatography, and mass spectroscopy because they hide the less abundant proteins of interest. High abundant proteins must be effectively, reproducibly, and extracted explicitly from blood samples to correctly detect the lower abundant proteins. The selective removal of albumin from human samples (e.g., plasma, serum, urine, or cerebrospinal fluid) is necessary before performing proteome analysis, particularly when using mass spectrometry.
Although Cibacron Blue F3GA, an affinity dye ligand, was covalently bonded onto commercially available supports for HSA adsorption from both aqueous solutions and human plasma, it lacks selectivity. In immunoaffinity systems, anti-albumin antibodies are also employed. These techniques have reasonable specificity in general, but they are costly. Furthermore, because many of these systems are based on agarose resin or its derived column, their procedure for the enrichment of the less abundant proteins is time-consuming, unable to handle large samples volume in a short time and challenging to adapt to the automation system. At the same time, they are frequently performed by increasing the sample volume, requiring an additional protein concentration step. New albumin removal methods with improved efficiency, homogeneity, and throughput are required to address the above issues.
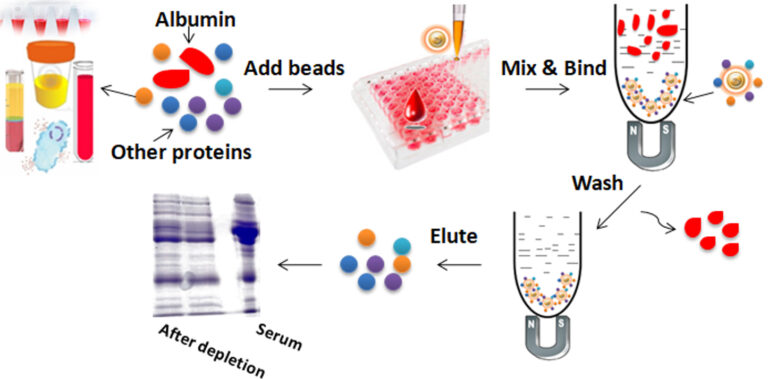
BcMag™ Quick Albumin Removal Kit is based on our unique magnetic beads modified with our proprietary chemistry for quick and easy albumin depletion from serum and plasma samples. The beads bind to all other proteins in the sample (except albumin) in particular buffer conditions, allowing the binding and release of all less abundant proteins but albumin. The enriched and eluted proteins can be used for downstream applications such as proteome analysis, biomarker detection, enzyme assays, SELDI analysis, protein array pixelation, 1D and 2D gel electrophoresis, LC/MS, and MALDI-TOF MS.
Workflow
Work with the Albumin Removal Kit is straightforward. Mix the beads with serum or plasma samples and incubate them with continuous rotation. The beads remain suspended in the sample solution during mixing, allowing all other proteins in the sample (except albumin) to bind to the beads. After incubation, the beads are collected and separated from the sample using a magnet rack. Then the bound proteins are eluted.
Features and Advantages
●
Quick, Easy, eliminates columns, centrifuge, or filters.
●
Remove >95% Albumin
●
High throughput: Rapidly process up to 96 samples in less than 30 minutes
●
Low abundance enrichment equal to or better than hexapeptides or antibodies
●
Mild elution preserves the tertiary structure and allows for easy transfer to secondary analysis
●
Scalable – easily adjusts for sample size and automation
●
Minimal sample dilution
●
Suitable for LC-MS, activity-based protein profiling, and proteomic research
PROTOCOL
Note
●
The following protocol is an example. Protein concentrations in serum might vary. Ten microliters of human serum have about 700µg of complete protein. About 70% of that will be human serum albumin. The amount of serum used in each response should be optimized by the user. Overloading the system may result in albumin carryover into the low abundant protein fraction. Using the given protocol, the user should expect a typical elution to include 50-100µg of serum protein.
●
The samples are eluted in a salt-containing buffer and may need to be desalted before electrophoresis or mass spectrometry analysis. If the sample is sufficiently diluted before analysis, desalting may not be required.
Materials Required
●
BcMag™ Quick Albumin Removal magnetic beads
●
1x Binding/Washing Buffer: 20 mM sodium phosphate, pH 6.5
●
1x Elution Buffer: 100 mM glycine-HCl, pH 2.7
Equipment
Item
Magnetic Rack for centrifuge tube
** Based on sample volume, the user can choose one of the following magnetic Racks
Source
• BcMag™ Rack-2 for holding two individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-01)
• BcMag™ Rack-6 for holding six individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-02)
• BcMag™ Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Bioclone, Cat. No. MS-03)
• BcMag™ Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-04)
Item
BcMag™ 96-well Plate Magnetic Rack.
Source
• BcMa™ 96-well Plate Magnetic Rack (side-pull) compatible with 96-well PCR plate and 96-well microplate or other compatible Racks (Bioclone, Cat. No. MS-05)
Item
Adjustable Single and Multichannel Pipettes
Item
Centrifuge with Swinging Bucket
Addition items are required if using 96-well PCR plates / tubes
Vortex Mixer
** The user can also use other compatible vortex mixers. However, the Time and speed should be optimized, and the mixer should be: Orbit ≥1.5 mm-4 mm, Speed ≥ 2000 rpm
Eppendorf™ MixMate™
Eppendorf, Cat. No. 5353000529
Tube Holder PCR 96
Eppendorf, Cat. No. 022674005
Tube Holder 1.5/2.0 mL, for 24 × 1.5 mL or 2.0 mL
Eppendorf, Cat. No. 022674048
Smart Mixer, Multi Shaker
BenchTop Lab Systems, Cat. No. 5353000529
1.5/2.0 mL centrifuge tube
96-well PCR Plates or 8-Strip PCR Tubes
PCR Plates/Tubes
** IMPORTANT! If using other tubes or PCR plates, make sure that the well diameter at the bottom of the conical section of PCR Tubes or PCR plates must be ≥2.5mm.
Items
Magnetic Rack for centrifuge tube
** Based on sample volume, the user can choose one of the following magnetic Racks
Source
●
BcMag™ Rack-2 for holding two individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-01)
●
BcMag™ Rack-6 for holding six individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-02)
●
BcMag™ Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Bioclone, Cat. No. MS-03)
●
BcMag™ Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-04)
BcMag™ 96-well Plate Magnetic Rack
●
BcMa™ 96-well Plate Magnetic Rack (side-pull) compatible with 96-well PCR plate and 96-well microplate or other compatible Racks (Bioclone, Cat. No. MS-05)
Adjustable Single and Multichannel Pipettes
Centrifuge with Swinging Bucket
Addition items are required if using 96-well PCR plates/tubes
Vortex Mixer
** The user can also use other compatible vortex mixers. However, the Time and Speed should be optimized, and the mixer should be: Orbit ≥1.5 mm-4 mm, Speed ≥ 2000 rpm
Eppendorf™ MixMate™
Tube Holder PCR 96
Tube Holder 1.5/2.0 mL, for 24 × 1.5 mL or 2.0 mL
Smart Mixer, Multi Shaker
Eppendorf, Cat. No. 5353000529
Eppendorf, Cat. No. 022674005
Eppendorf, Cat. No. 022674048
BenchTop Lab Systems, Cat. No. 5353000529
Eppendorf™ MixMate™
Tube Holder PCR 96
Tube Holder 1.5/2.0 mL, for 24 × 1.5 mL or 2.0 mL
Smart Mixer, Multi ShakerEppendorf, Cat. No. 5353000529
Eppendorf, Cat. No. 022674005
Eppendorf, Cat. No. 022674048BenchTop Lab Systems, Cat. No. 5353000529
1.5/2.0 mL centrifuge tube
96-well PCR Plates or 8-Strip PCR Tubes
PCR Plates/Tubes
! IMPORTANT ! If using other tubes or PCR plates, ensure that the well diameter at the bottom of the conical section of PCR Tubes or PCR plates must be ≥2.5mm.
Procedure
Note:
●
Vigorously shake the bottle until the magnetic beads become homogeneous.
●
Do not allow the magnetic beads to sit for more than 5 minutes before dispensing.
1.
Transfer 25 μl beads to a fresh tube or a new 96well PCR plate, Microplate, or 0.2ml PCR tube and add 65 µl binding buffer to a final 90 μL reaction volume.
2.
Add 10 μl serum sample and mix the sample with beads by slowly pipetting up and down 20-25 times, or Vortex the sample at 2000 rpm for 5 minutes (see picture).

3.
Place the sample plate/ tube on the magnetic separation plate for 30 seconds or until the solution is clear.
4.
Discard the supernatant while the sample plate remains on the magnetic separation plate.
5.
Wash the beads with 100 μL of the binding buffer by slowly pipetting up and down 20-25 times, or Vortex the sample at 2000 rpm for 2 minutes. Place the sample plate/ tube on the magnetic separation plate for 30 seconds or until the solution is clear.
6.
Repeat step 5 once.
7.
Resuspend the beads in 50-100μl of Elution Buffer and mix the sample with beads by slowly pipetting up and down 20-25 times, or Vortex the sample at 2000 rpm for 5 minutes.
8.
Transfer the supernatant to a fresh microcentrifuge tube or microtiter plate well. The supernatant containing the proteins of interest is ready for downstream applications.
Learn More
Instruction Manual
MSDS
Sample Preparation Related Products →


