
Ethylene glycol tetraacetic acid (EGTA) is a chelating agent and a polycarboxylic amino acid. EGTA has a stronger affinity for calcium ions but a lower affinity for magnesium ions than EDTA. EGTA, like EDTA, can be used as a buffer to simulate the pH of a living cell. Because of this feature, EGTA can be used in Tandem Affinity Purification, a protein purification process. EGTA has a higher boiling point than EDTA.
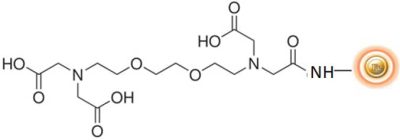
BcMag™ EGTA Metal Ion Removal Kit uses magnetic beads coated with high-density EGTA (Ethylene glycol tetraacetic acid) functional groups on the surface. Compared to EDTA, EGTA has a higher affinity for calcium ions but a lower affinity for magnesium ions. The EGTA beads are an ideal tool for removing the metal ions such as Ca, As, Gd, La, Ni, Se, Zn, Co, Fe, Mg, Pd, Pt & Rh, etc.
Features and Advantages
●
Quick, Easy, and one-step high-throughput procedure to chelate metal ions;
Eliminates columns or filters, and a laborious repeat of pipetting or centrifugation
●
High capacity
●
Reproducible results
PROTOCOL
Magnetic Beads Preparation
1.
Suspend the bead with dH2O to 30mg/ml concentration.2.
Transfer the desired amount of magnetic beads to a centrifuge tube.
3.
Place the tube on the magnetic separator for 1-3 minutes. Remove the supernatant while the tube remains on the separator. Remove the tube and resuspend the beads thoroughly with dH2O. Place the tube on the magnetic separator for 1-3 minutes. Remove the supernatant while the tube remains on the separator.4.
The beads are ready to use.
Learn More
Instruction Manual
MSDS
Sample Preparation Related Products →


