
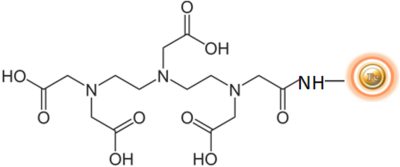
EDTA is an abbreviation for ethylene-diamine-tetraacetic acid. EDTA is a polyprotic acid with four carboxylic acid groups and two amine groups with lone pair electrons. It is frequently used to chelate divalent cations and is widely utilized in biochemistry and molecular biology.
BcMag™ EDTA Metal Ion Removal Magnetic Beads use magnetic particles coated with high-density EDTA (Ethylene Diamine Triacetic Acid) functional groups on the surface to remove metals. The beads chelate metal ions such as As, Ca, Cu, Gd, La, Ni, Se, Zn, Co, Fe, Mg, Pd, Pt & Rh, etc. The beads are an excellent tool for removing the divalent cations from the buffer.
Features and Advantages
●
Quick, Easy, and one-step high-throughput procedure to chelate metal ions;
Eliminates columns or filters, and a laborious repeat of pipetting or centrifugation
●
High capacity
●
Reproducible results
PROTOCOL
Magnetic Beads Preparation
1.
Shake the bottle to resuspend the EDTA beads thoroughly.
2.
Transfer the desired amount of magnetic beads to a centrifuge tube.
3.
Place the tube on the magnetic separator for 1-3 minutes. Remove the supernatant while the tube remains on the separator. Remove the tube and resuspend the beads thoroughly with dH2O. Place the tube on the magnetic separator for 1-3 minutes. Remove the supernatant while the tube remains on the separator.4.
Repeat step 3 once.
5.
The beads are ready to use.
Learn More
Instruction Manual
MSDS
Sample Preparation Related Products →


