
Fluorescent dyes, also called reactive dyes or fluorophores, are natural or synthetic compounds that absorb light and re-emit it at a longer wavelength. Due to their unique advantages such as versatility, sensitivity, and quantitative capabilities, fluorescent dyes are widely used to label biologically relevant molecules such as protein, antibodies, peptides, DNA, and RNA for their applications in cell biology, immunology, biochemistry, microbiology, molecular biology, genomics, and proteomics. After a fluorescent labeling reaction, removing excess or unreacted dye from final conjugates is often necessary since it interferes with many downstream applications. Removing fluorescent dyes is usually accomplished by spin columns, gel filtration, gravity-flow columns, and dialysis. However, those traditional methods present many problems, including time-consuming and labor-intensive processes, poor recovery of protein, peptides, or nucleic acids, and the challenge of adapting to automation. For this reason, we introduce a novel one-minute dye removal system.
BcMag™ One-Step Dye Removal Kit uses specially formulated resin with a proprietary surface chemistry to specifically remove the excess free (non-conjugated) fluorescent dyes from the finished protein, peptide or antibody labeling reaction. Compared with the dye removal columns, the resin can quickly and efficiently remove free dyes from the sample with just a single step and enables an individual or 96 samples to be processed simultaneously in less than 1 or 10 minutes with very little hands-on time (Fig.1). Since the magnetic resin only adsorbs the free dye, the labeled biomolecule recovery rate is exceptionally higher than >90%. Moreover, the magnetic beads can remove most of the dyes if the appropriate amount of samples and buffer conditions are used.
Workflow
The one-minute dye removal protocol is straightforward.
1.
Add the beads directly to the sample.
2.
Pipette or vortex to capture the free dye.
3.
Magnetic separation of the beads from the protein solution, while the supernatant contains the purified and ready-to-run products.
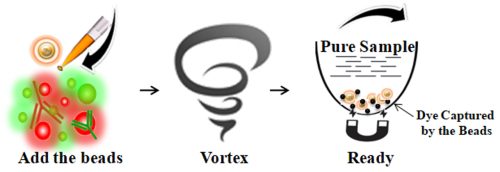
Features and Advantages
●
Simple protocol: No liquid transfer, One-tube, One-step, and One-minute protocol
●
Easy-to-use
●
Reliable and reproducible results with exceptional >90% recovery for protein (>6 kDa, aprotinin) or DNA/RNA (>25mer dsDNA)
●
Effective Cleanup: Remove 95% free dye
●
Cost-effective: Eliminates columns, filters, and laborious repeat pipetting
●
High-throughput: Compatible with many different automated liquid handling systems
PROTOCOL
Materials Required
Item
Magnetic Rack for centrifuge tube
** Based on sample volume, the user can choose one of the following magnetic Racks
Source
• BcMag™ Rack-2 for holding two individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-01)
• BcMag™ Rack-6 for holding six individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-02)
• BcMag™ Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Bioclone, Cat. No. MS-03)
• BcMag™ Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-04)
Item
BcMag™ 96-well Plate Magnetic Rack.
Source
• BcMa™ 96-well Plate Magnetic Rack (side-pull) compatible with 96-well PCR plate and 96-well microplate or other compatible Racks (Bioclone, Cat. No. MS-05)
Item
Adjustable Single and Multichannel Pipettes
Item
Centrifuge with Swinging Bucket
Addition items are required if using 96-well PCR plates / tubes
Vortex Mixer
** The user can also use other compatible vortex mixers. However, the Time and speed should be optimized, and the mixer should be: Orbit ≥1.5 mm-4 mm, Speed ≥ 2000 rpm
Eppendorf™ MixMate™
Eppendorf, Cat. No. 5353000529
Tube Holder PCR 96
Eppendorf, Cat. No. 022674005
Tube Holder 1.5/2.0 mL, for 24 × 1.5 mL or 2.0 mL
Eppendorf, Cat. No. 022674048
Smart Mixer, Multi Shaker
BenchTop Lab Systems, Cat. No. 5353000529
1.5/2.0 mL centrifuge tube
96-well PCR Plates or 8-Strip PCR Tubes
PCR plates/tubes
** IMPORTANT! If using other tubes or PCR plates, make sure that the well diameter at the bottom of the conical section of PCR Tubes or PCR plates must be ≥2.5mm.
Items
Magnetic Rack for centrifuge tube
** Based on sample volume, the user can choose one of the following magnetic Racks
Source
●
BcMag™ Rack-2 for holding two individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-01)
●
BcMag™ Rack-6 for holding six individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-02)
●
BcMag™ Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Bioclone, Cat. No. MS-03)
●
BcMag™ Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-04)
BcMag™ 96-well Plate Magnetic Rack
●
BcMa™ 96-well Plate Magnetic Rack (side-pull) compatible with 96-well PCR plate and 96-well microplate or other compatible Racks (Bioclone, Cat. No. MS-05)
Adjustable Single and Multichannel Pipettes
Centrifuge with Swinging Bucket
Addition items are required if using 96-well PCR plates/tubes
Vortex Mixer
** The user can also use other compatible vortex mixers. However, the Time and Speed should be optimized, and the mixer should be: Orbit ≥1.5 mm-4 mm, Speed ≥ 2000 rpm
Eppendorf™ MixMate™
Tube Holder PCR 96
Tube Holder 1.5/2.0 mL, for 24 × 1.5 mL or 2.0 mL
Smart Mixer, Multi Shaker
Eppendorf, Cat. No. 5353000529
Eppendorf, Cat. No. 022674005
Eppendorf, Cat. No. 022674048
BenchTop Lab Systems, Cat. No. 5353000529
Eppendorf™ MixMate™
Tube Holder PCR 96
Tube Holder 1.5/2.0 mL, for 24 × 1.5 mL or 2.0 mL
Smart Mixer, Multi ShakerEppendorf, Cat. No. 5353000529
Eppendorf, Cat. No. 022674005
Eppendorf, Cat. No. 022674048BenchTop Lab Systems, Cat. No. 5353000529
1.5/2.0 mL centrifuge tube
96-well PCR Plates or 8-Strip PCR Tubes
PCR Plates/Tubes
! IMPORTANT ! If using other tubes or PCR plates, ensure that the well diameter at the bottom of the conical section of PCR Tubes or PCR plates must be ≥2.5mm.
Procedure
! IMPORTANT !
●
The following protocol is an example. The beads and sample volume can be rational scale-up (or down). Do not use buffers containing organic solvents.
●
The user should optimize the beads and detergent concentration ratio based on the binding capacity, as the examples listed in Table 1. Some dyes may require double processing because they require high molar excesses for labeling.
Table 1. Dye Binding Capacity
Fluorescent Dyes
Binding Capacity
ng /mg beads**
Alexa Fluor 546 C5-Maleimide
99.7
Alexa Fluor™ 514 NHS Ester
45.2
Cyanine 3 carboxylic acid
99.1
Cyanine 5 carboxylic acid
49.7
Cyanine 3 amine
99.3
Cyanine 3.5 carboxylic acid
99
Cyanine 5.5 amine
99.8
Cyanine 5.5 carboxylic acid
99.7
Cyanine 5 amine
49.85
Sulfo-Cyanine 5.5 amine
99.9
Sulfo-Cyanine3 amine
93.3
Sulfo-Cyanine5 carboxylic acid
24.9
DyLight™ 488 NHS Ester
90.5
DyLight™ 633 NHS Ester
87.4
Dylight 680-4x PEG NHS Ester
99.8
DyLight™ 405 NHS Ester
99
Oregon Green™ 488 carboxylic acid
84.2
FAM amine, 5-isomer
24.57
Rhodamine 5B amine
99.2
Texas Red™ hydrazide
890
Cibarcron blue F3GA
99.7
Fluorescein isothiocyanate
120.3
Bromocresol purple
105.2
Phenol red
99.5
Denim red
101
Bromophenol blue
99
Denim blue
104.2
SYBR® dye102.4
Fluorescent Dyes
Binding Capacity
ng /mg beads**
Fluorescent dyes
Binding capacity
ng /mg beads**
Alexa Fluor 546 C5-Maleimide
99.7
Alexa Fluor™ 514 NHS Ester
45.2
Cyanine 3 carboxylic acid
99.1
Cyanine 5 carboxylic acid
49.7
Cyanine 3 amine
99.3
Cyanine 3.5 carboxylic acid
99
Cyanine 5.5 amine
99.8
Cyanine 5.5 carboxylic acid
99.7
Cyanine 5 amine
49.85
Sulfo-Cyanine 5.5 amine
99.9
Sulfo-Cyanine3 amine
93.3
Sulfo-Cyanine5 carboxylic acid
24.9
DyLight™ 488 NHS Ester
90.5
DyLight™ 633 NHS Ester
87.4
Dylight 680-4x PEG NHS Ester
99.8
DyLight™ 405 NHS Ester
99
Oregon Green™ 488 carboxylic acid
84.2
FAM amine, 5-isomer
24.57
Rhodamine 5B amine
99.2
Texas Red™ hydrazide
890
Cibarcron blue F3GA
99.7
Fluorescein isothiocyanate
120.3
Bromocresol purple
105.2
Phenol red
99.5
Denim red
101
Bromophenol blue
99
Denim blue
104.2
SYBR® dye102.4
**Assay condition: Mix 10 µl magnetic beads (100 mg/ml) with 100 µl protein sample (1:400 dilution of Human serum) containing different dyes at a concentration of 50 ng-800 ng in 0.1M Sodium phosphate, 0.15M NaCl, pH7.5 buffer, and vortex at 2000 rpm for 5 minutes. The dye removal efficiency is >93%, while protein recovery is >95%.
1.
Adjust the sample pH to 6.5 to 9.0 and the NaCl concentration to 150 mM.
2.
Shake the bottle to resuspend the Magnetic beads until it is homogeneous entirely.
! IMPORTANT !
- It is essential to mix the beads before dispensing.
- Do not allow the beads to sit for more than 2 minutes before dispensing. Resuspend the magnetic beads every 2 minutes.
3.
Add 10µl magnetic beads to a 100 µl sample containing free dyes.
! IMPORTANT !
Users need to optimize the ratio of beads and free dyes since the free dye concentration varies from sample to sample due to the dye labeling efficiency.
4.
Mix the sample with beads for 1-2 minutes by slowly pipetting up and down 20-25 times, or vortex the sample for 5 minutes at 2000 rpm (PCR plate or tube) or 800 rpm (96-well microplate).
! IMPORTANT !
Users need to optimize time and speed if using a vortex mixer.
5.
Place the sample plate or tube on the magnetic separation plate for 30 seconds or until the solution is clear.
6.
Transfer the supernatant to a clean plate /tube while the sample plate remains on the magnetic separation plate. The sample is ready for downstream applications.
C. Troubleshooting
Problem
Low Protein Recovery
Probable Cause
Vortexing time is too long.
Suggestion
If using other digital vortex mixers, the vortex conditions such as speed and time must be optimized.
Problem
Low Protein Recovery
Probable Cause
Using too many magnetic beads
Suggestion
Thoroughly resuspend the magnetic beads and reduce the amounts of the beads.
Problem
Failure to Remove Dye
Probable Cause
Used inappropriate tubes or plates
Suggestion
Make sure that the well diameter at the bottom of the conical section of the Tubes or well of the plate is ≥2.5mm.
Problem
Failure to Remove Dye
Probable Cause
- Vortex speed is too slow, or vortex time is too short.
- Containing too much free bye in the sample
Suggestion
- Increasing either the speed or time
- If using other digital vortex mixers, the vortex condition such as speed and time must be optimized.
- Repeat the procedure using more beads
Problem
Probable Cause
Suggestion
Low Protein Recovery
Vortexing time is too long.
If using other digital vortex mixers, the vortex condition such as speed and time must be optimized.
Using too many magnetic beads
Thoroughly resuspend the magnetic beads and reduce the amounts of the beads.
Failure to remove dye
Used inappropriate tubes or plates
Make sure that the well diameter at the bottom of the conical section of the Tubes or well of the plate is ≥2.5mm.
- Vortex speed is too slow, or vortex time is too short.
- Containing too much free dye in the sample
- Increasing either the speed or time
- If using other digital vortex mixers, the vortex condition such as Speed and Time must be optimized.
- Repeat the procedure using more beads



