
Plasmids are circular, extrachromosomal DNA molecules that act as carriers, or vectors, for a specific DNA segment in molecular biology. Bacteria are employed to reproduce plasmids, allowing your desired DNA to be mass-produced. The method by which researchers acquire plasmids from bacteria is known as plasmid purification.
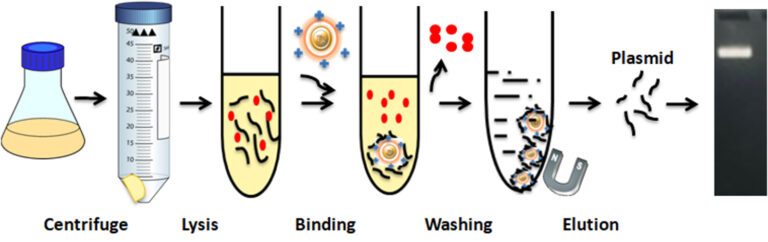
Purifying plasmid DNA from bacterial cells is a critical step in cloning. Bacterial cells are lysed during plasmid purification, which liberates DNA and other biological components from the cell wall. After removing cellular components, the DNA-containing lysate is treated to eliminate impurities and separate the plasmid DNA from the genomic DNA.
The plasmid DNA extracted is the same quality as that produced by purification with two passes through cesium chloride gradients—the most stringent approach for DNA purification. High-quality DNA can be purified in less than twenty minutes for transfections and other applications. There is no need for extra processes to remove impurities such as RNA, proteins, or endotoxins. Furthermore, the protocol’s exclusion of phenol, chloroform, ethidium bromide, and cesium chloride reduces exposure to and disposal of hazardous compounds.
Principle & Workflow
BcMag™ Pure Midiprep Plasmid DNA Purification uses weak anion exchange beads to quickly purify plasmid DNA. The beads allow for plasmid DNA binding directly. This approach uses typical cell resuspension, alkaline lysis, and neutralization processes and allows plasmid DNA to bind directly to the beads. A single bead wash removes impurities such as RNA, proteins, metabolites, and other low molecular weight molecules. Unique wash buffers ensure that salts, proteins, RNA, and other biological components are eliminated, allowing concentrated, highly pure DNA to be eluted. The ultrapure plasmid DNA is subsequently eluted with a high-salt buffer. The DNA is desalted, concentrated with an isopropanol precipitation process, and centrifuged. The entire treatment takes roughly twenty minutes to complete.
Feature and Benefits
●
Simple protocols- no columns or filters required.
●
Quick results- purify plasmid DNA in less than twenty minutes.
●
No Phenol/chloroform extractions
●
Low endotoxin
●
Quality – DNA is suitable for most downstream applications
●
Scalable – Easily adjusts for sample size and automation
●
Reproducible results
Specificities
Composition
Weak anion exchange beads
Magnetization
~45 EMU/g
Type of Magnetization
Superparamagnetic
Beads concentration
2.0 g/ml
Concentration
50mg/ml (ultrapure water)
Binding Capacity
~500µg DNA / ml beads
Storage
Store at 4°C upon receipt
PROTOCOL
Materials Required
A.
Buffer Composition
●
Material Required: BcMag™ Plasmid DNA Magnetic Beads
●
TE Buffer: 10mM Tris.HCL PH8.0 1mM EDTA
●
Isopropanol (Sigma Cat. No. 19516)
●
P1 (Resuspension buffer): 50mM Tris.HCl, pH 8.0, 10mM EDTA, 100ug/ml RNase A (Store at 4°C after addition of RNase A)
●
P2 (lysis buffer): 200mM NaOH, 1% SDS (w/v)
●
P3 (Neutralization buffer): 3.1M Potassium Acetate, pH 5.5 (Store at 4°C)
●
P4 ( Equilibration buffer): 750mM NaCl, 50mM MOPS, pH 7.0, 15% isopropanol (v/v), 0.15% Triton X-100 (v/v)
●
P5 (Wash buffer): 1.0 M NaCl, 50mM MOPS PH 7.0, 15% isopropanol(v/v)
●
P6 (Elution buffer): 2.5 M NaCl, 50mM Tris.HCl, pH8.5, 15% isopropanol (v/v)
B.
Equipments
●
Magnetic Rack (for manual operation)
Based on sample volume, the user can choose one of the following magnetic Racks:
– BcMag™ Rack-2 for holding two individual 1.5 ml centrifuge tubes (Cat. No. MS-01);– BcMag™ Rack-6 for holding six individual 1.5 ml centrifuge tubes (Cat. No. MS-02);– BcMag™ Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Cat. No. MS-03);– BcMag™ Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Cat. No. MS-04);– BcMag™ Rack-96 for holding a 96 ELISA plate or PCR plate (Cat. No. MS-05).For larger scale purification, Ceramic magnets Block for large scale purification (6 in x 4 in x 1 in block ferrite magnet, Applied Magnets, Cat. No. CERAMIC-B8)
●
Corning 430825 cell culture flask for large-scale purification (Cole-Parmer, Cat. No. EW-01936-22)
●
Mini BlotBoy 3D Rocker, fixed speed, small 10″ x 7.5″ platform w/ flat mat (Benchmark Scientific, Inc. Cat. No. B3D1008) or compatible
C.
Procedure
** The protocol is based on a 50 ml bacterial culture with a high copy plasmid. However, the protocol can be properly scaled up or down based on bacterial culture volume.
1.
Bacterial lysis
a.
Transfer 50ml overnight cell culture to a centrifuge tube, centrifuge for 10 minutes at rpm 6000, and remove the supernatant.
b.
Completely resuspending bacterial pellets with 4ml of P1 buffer, add 4ml of P2 buffer mix gently by inverting and incubating at room temperature for 5 min.
c.
Add buffer 4ml of P3, mix gently by inverting, centrifuge at 12000-16000 rpm for 10 min at room temperature, and carefully transfer the supernatant. (if necessary, filter the supernatant through six layers of cheesecloth) to a fresh tube.
2.
Plasmid purification
a.
Vigorously shake the bottle until the magnetic resins become homogeneous, and transfer 1 ml of beads to a fresh tube.
Note: Do not allow the resins to sit for more than 3 minutes before dispensing. Resuspend the magnetic beads every 3 minutes.
b.
Place the tube on the magnetic Rack for 1-3 minutes. Remove the supernatant while the tube remains on the Rack. Add ten bead-bed volumes of P4 buffer, and mix the beads by pipetting or vortex. Again, place the tube on the magnetic Rack for 1-3 minutes and remove the supernatant while the tube remains on the Rack.
c.
Mix the beads with cell lysate (step1c), incubate on Mini BlotBoy 3D Rocker with continuous rotation for 5-10 minutes at room temperature, and place the tube on a Rack for 1 minute until it becomes clear, and then remove supernatant.
d.
Wash the magnetic bead with 6ml of P5 buffer three times using a magnetic Rack.
e.
Add 1ml elution buffer to the magnetic beads and completely resuspend the beads by pipetting up and down 15-20 times. Place the tube on a Rack for 1-3min and transfer the supernatant to a new tube.
3.
Plasmid precipitation
a.
Add 700ul isopropanol, mix well, incubate at room temperature for 2 minutes, and centrifuge at 14000-16000 rpm for 10 minutes at room temperature.
b.
Carefully remove the supernatant, wash pellets with 600ul of 70% ethanol, centrifuged at 14000-16000 rpm for 4 minutes.
c.
Carefully remove the supernatant and air dry the pellet for 5-10 minutes. (**Do not overdry the pellet. Or the pellet is difficult to be resolved).
d.
Resuspend the pellet with the desired amount of TE buffer. (**Run 2-3 ul of plasmid solution on 1% agarose gel to check the quality. If RNA is present, add RNAse A to completely remove the RNA)
Learn More
Instruction Manual
MSDS
DNA & RNA Purification Related Products →


