
PCR technology has become one of the most potent molecular biological tools in the last few decades. However, biological samples collected from different materials often contain various PCR inhibitors that can interfere with PCR amplification. PCR inhibitors are a diverse group of compounds that inhibit the amplification of DNA through the polymerase chain reaction (PCR). PCR inhibitors generally exert their effects by directly binding the active site of a DNA polymerase to cause decreased sensitivity or complete failures of the DNA amplification. Therefore, removing PCR inhibitors from the DNA extracts before the PCR amplification is vital for all downstream applications.
Where Do the PCR inhibitors originate?
PCR inhibitors exist in a variety of biological materials (organs, blood, body fluids, etc.), environmental samples (water, soil, air, etc.), and food (meat, milk, fruits, vegetables, seafood, etc.). In addition, inhibitory substances may be accidentally added during transport, sample processing (e.g., pre-concentration procedures), or nucleic acid extraction. PCR inhibitors can come from the original sample themself, sample preparation, DNA purification process, or dirty plasticware. Table1 shows some examples of sources and their specific inhibitors. Table 1 lists the common PCR inhibitors.
Table1 - PCR Inhibitor
Sources
Blood, Muscle tissues
Inhibitor
Heparin, Hemoglobin, Immunoglobulins, Proteases, Nucleases, Acyclovir, Hormones, IgG, Lactoferrin, Myoglobin, Collagen
Milk
Proteases, Calcium ions
Stool
Plants
Soil
Sample preparation
DNA purification
Dirty plasticware
Bile salts, Complex polysaccharides, Lipids, Urate
Pectin, Polyphenols, Polysaccharides, Xylan, Chlorophyll
Fulmic acids, Humic acids, Humic material, Metal ions, Polyphenol
KCl, SDS, Xylene
Chaotropic salts, Ethanol, Isopropanol, Phenol, Sodium acetate
Protease, Nucleases
PCR Inhibitor
[Sources]Blood, Muscle Tissues[Inhibitor]
Heparin, Hemoglobin, Immunoglobulins, Proteases, Nucleases, Acyclovir, Hormones, IgG, Lactoferrin, Myoglobin, Collagen[Sources]
Milk[Inhibitor]
Proteases, Calcium ions[Sources]
Stool[Inhibitor]
Bile salts, Complex polysaccharides, Lipids, Urate[Sources]
Plants[Inhibitor]
Pectin, Polyphenols, Polysaccharides, Xylan, Chlorophyll[Sources]
Soil[Inhibitor]
Fulmic acids, Humic acids, Humic material, Metal ions, Polyphenol[Sources]
Sample Preparation[Inhibitor]
KCl, SDS, Xylene[Sources]
DNA Purification[Inhibitor]
Chaotropic salts, Ethanol, Isopropanol, Phenol, Sodium acetate[Sources]
Dirty Plasticware[Inhibitor]
Protease, NucleasesPrevious slideNext slide
How to Remove the PCR Inhibitors?
Several methods have been used for the removal of inhibitors or the reduction of their effects –
1.
Dilute the sample extracts containing PCR inhibitors. It is simple, but the diluted DNA may not be enough for successful DNA amplification, and a decrease in sensitivity accompanies the dilution.
2.
Chelex such as Chelex1-100 and Phenol–Chloroform-based protocols. Both methods are inefficient for removing most of the PCR inhibitors since Chelex can only remove some divalent ions. At the same time, the Phenol–Chloroform method can only deplete lipids and proteins.
3.
Column chromatography such as Sephacryl S-400, Sephadex G-200, and silica-based spin-column or magnetic beads effectively remove some of the inhibitors, but the process is labor-intensive and time-consuming.
In summary, although these methods can remove some PCR inhibitors, however, practically they have the following limitations such as:
1.
Less efficient, time-consuming, or labor-intensive.
2.
Potential loss of DNA sample during processing.
3.
Chaotropic salt and ethanol are potentially carried over into the eluted DNA.
4.
They are not suitable for high-throughput processing and automation.
For this reason, Bioclone developed a novel, highly efficient PCR inhibitor removal system based on magnetic beads –
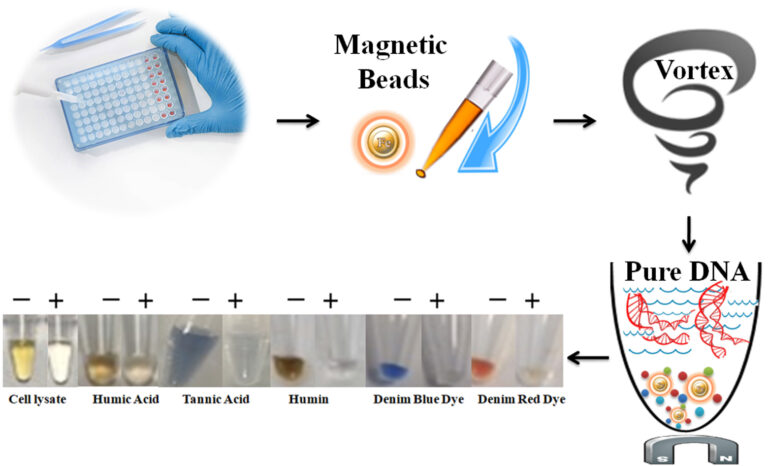
BcMag™ One-Step PCR Inhibitor Removal Kit provides one-step removal of PCR inhibitor from impure DNA samples before PCR, RT, and other downstream applications based on negative chromatography. The magnetic beads are superparamagnetic and modified with our proprietary chemistry. When mixed with inhibitor-containing samples, the beads instantly capture and remove the PCR inhibitors. At the same time, only the pure DNA remains in the solution and is ready for all downstream applications (Fig.1). The beads can effectively remove many common inhibitors such as polyphenolic compounds, humic/fulvic acids, acidic polysaccharides, tannins, melanin, heparin, detergents, and denim dyes, and divalent cations such as Ca2+, Mg2+, etc.
Workflow
The protocol is straightforward and fast: one tube, one step, and one minute (Fig.1). Add the magnetic beads directly to the pre-purified DNA samples and vortex or pipette to capture and remove the impurities. After vortexing/pipetting, the beads are magnetically removed, while the supernatant contains the purified and ready-to-run products. Unlike standard bind-wash-elute protocol, this convenient procedure does not contain traces of organic solvents, chaotropic salts, or EDTA and is almost 100% DNA recovery. The beads enable 96 samples to be processed simultaneously in less than 10 minutes with cost-effective lab vortex mixers.
Features and Advantages
●
Simple Protocol: No liquid transfer, One-tube, One-step
●
Ultrafast: One-minute manual protocol or less than 10-minutes vortex (96 samples)
●
Higher purity and recovery > 90% DNA (> 50bp)
●
Effective Cleanup: polyphenolic compounds, humic/fulvic acids, acidic polysaccharides, tannins, melanin, detergents, divalent cations such as Ca2+, Mg2+, etc.●
Cost-effective: Eliminates columns, filters, laborious repeat pipetting, and ethanol
●
High throughput: Compatible with many different automated liquid handling systems
●
Handling and Storage:
- Store at 4°C upon arrival for up to 12 months.
- DO NOT FREEZE
Learn More
Instruction Manual
MSDS
DNA & RNA Purification Related Products →


